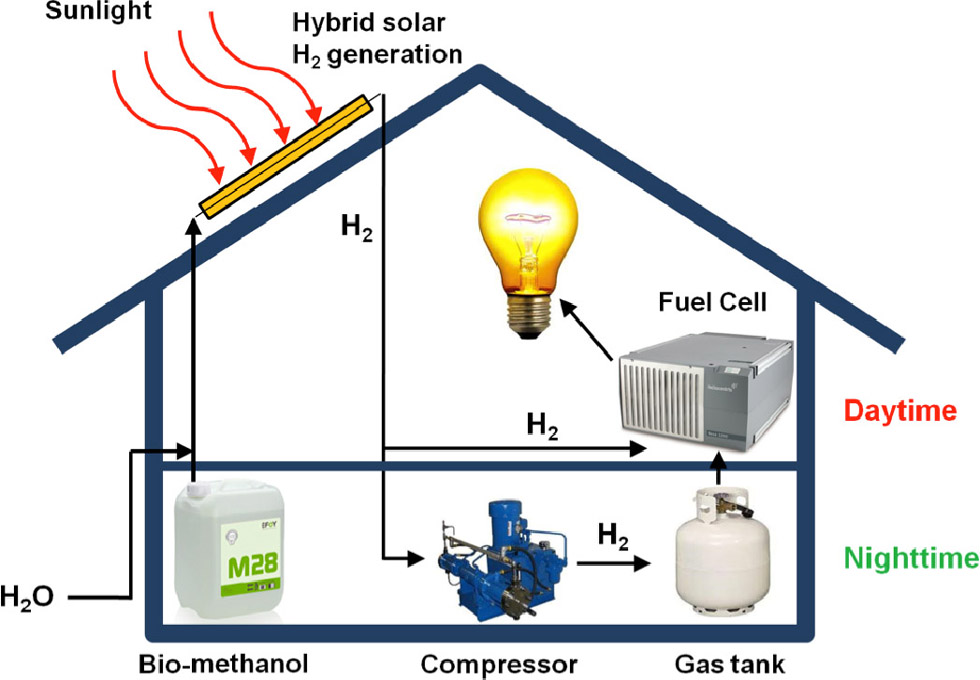
Producing hydrogen on the rooftop from sunlight and biofuel
Renewable energy is the ultimate way to fulfill human demand for power in a sustainable, carbon-neutral, environmentally benign, and clean way. Researchers are studying both photovoltaic (solar cell) devices to convert solar power to electricity and fuel cells to generate electricity from hydrogen. Photovoltaics suffers from low solar-to-electric energy efficiencies (typically between 10 and 15% for commercially available systems) and high installation costs. Low-temperature fuel cells fed by hydrogen offer several advantages for generating electrical power, namely, high energy and power densities per mass and volume, high energy-conversion efficiencies, instantaneous recharging, and fast response to load changes. The most popular of these devices is the proton-exchange membrane (PEM) fuel cell, which is already commercially available and can be operated at temperatures typically between 60 and 90°C. However, due to the low energy density of hydrogen per volume, the storage of hydrogen within a relatively small fuel cell system in a building for long-term use is not only technically difficult but also very cumbersome and bulky. We are currently investigating the possibility of combining sunlight harvesting with hydrogen generation from a biofuel to produce electricity for residential and non-residential buildings (see Figure 1).
Our approach involves conversion of methanol to hydrogen (so-called methanol steam reforming), PEM fuel cells, and solar collectors to generate the required heat for the steam reforming. The synergies of these technologies lead to a highly efficient system with significantly greater power densities compared with conventional systems and tremendous advantages in terms of installation and operation costs.
Due to its reasonable energy density, very simple storage, easy availability, and especially the possibility to generate it from biomass, methanol is a highly appropriate fuel for a fuel cell system containing a methanol-to-hydrogen reformer.1 Methanol steam reforming on a copper-based catalyst can be performed with high efficiency, resulting in a reformate gas containing up to 75% hydrogen. Because of the chemical nature of the steam-reforming reaction, the reactor must be externally heated to reach and maintain the necessary reaction temperature. Our method provides the required heat to the system in the form of solar energy harvested by a solar thermal collector. The efficiency of such a mini power plant fed by methanol can be dramatically increased if solar energy is used as a heat source instead of other external heaters, e.g., fuel-consuming burners.
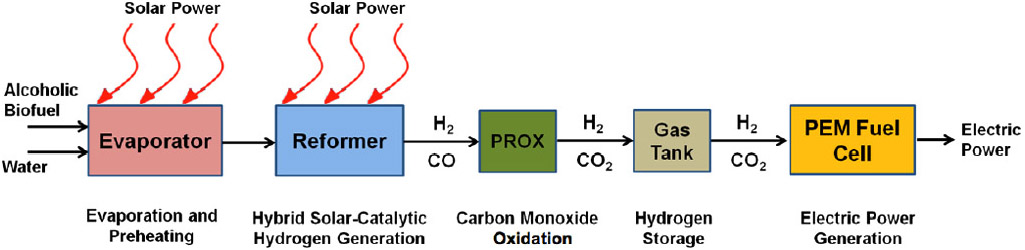
In a recent computational study,2 the hybrid solar system was shown to achieve significant advantages both in terms of efficiency and cost for typical conditions found in northern California (San Francisco Bay Area) when considering the conversion of sunlight and methanol to hydrogen and consequently the conversion of hydrogen to electricity in a fuel cell. Figure 2 shows schematically how the entire system works. First, the incoming mixture of liquid alcoholic fuel and water is evaporated and preheated to the reaction temperature by solar power. Typically, this reaction in the reformer takes place at temperatures between 200 and 250°C, resulting in a reformer product gas containing up to 75% hydrogen. Because the reformate gas contains a small amount of carbon monoxide, which is poisonous to the fuel cell, it is removed in a preferential oxidation (PROX) reactor following the reformer. The final gas mixture can be used directly in the PEM fuel cell or stored temporarily in a pressurized gas tank.
Direct solar-to-electric energy conversion, as occurs with photovoltaics, is currently not economically competitive with traditional electric power generation. Fuel cell technology that uses alcoholic fuel directly, possibly generated from biomass (e.g., methanol), is not cost-effective, either. The system proposed here, in contrast, consists of relatively cheap, commercially available hardware components (intermediate-temperature solar collector, pressurized gas tank, hydrogen-fed PEM fuel cell) and has the advantage of energy efficiency from the additional use of solar heat. The storage of liquid methanol as primary fuel in a simple and safe tank requires an order of magnitude less space than a hydrogen gas tank.
Moreover, the hybrid system with hydrogen as an intermediate product benefits from the efficient short-term storage of a limited amount of hydrogen in a pressurized tank. When the energy consumption of a compressor for storing pressurized hydrogen is taken into account, the energy efficiency of the gas storage amounts to about 94%.3 This allows for the temporary storage of energy in the system, avoiding the need for expensive and inefficient electric energy storage, e.g., batteries, with efficiencies of around 65% for nickel-cadmium and 75% for lead-acid types.4 In fact, the only batteries that can compete with our proposed system are the more expensive lithium-ion batteries, which achieve 95% efficiency on average.4 The on-site energy storage leads to a more controllable and highly flexible demand response since the stored hydrogen can be almost instantaneously converted to electric power by the fuel cell. This system configuration solves the problem of unbalanced power supply by sunlight during day and night, an intrinsic disadvantage of solar power. The system can be operated without relying on power from the electric grid during peak hours in the early evening, as is typical for residential buildings, when power demand is high but incoming solar power already low.
In summary, we have described a novel approach that combines a solar-heated methanol steam reformer with a PEM fuel cell to achieve fuel-to-electricity efficiencies of ∼50%, electric power densities of close to 1000W/m2 per irradiated area, and total costs per energy unit on the order of $0.17/kWh.2, 3,5 As the next step, we plan to work on a demonstrator system to confirm the efficiency and performance of the proposed solution experimentally.
Nico Hotz received his MSc and PhD in mechanical engineering from the Swiss Federal Institute of Technology Zurich, Switzerland (2005 and 2008). After postdoctoral research at the University of California, Berkeley, he became assistant professor in the Department of Mechanical Engineering and Materials Science at Duke University, where he heads the Thermodynamics and Sustainable Energy Laboratory.