With the discovery of x-ray in 1895, images are routinely acquired for medical diagnostics. Fostered by the increasing use of direct digital imaging systems, digital image processing has become increasingly important in health care. In addition to originally digital methods such as computed tomography (CT) or magnetic resonance imaging (MRI), initially analogue imaging modalities such as endoscopy or radiography have now been equipped with digital sensors.
Digital images are composed of individual pixels (this acronym is formed from the words "picture" and "element"), where discrete brightness or color values are assigned. They can be efficiently processed, objectively evaluated and made available on many places at the same time by means of appropriate communication networks and protocols, such as picture archiving and communication systems (PACS) and the digital imaging and communications in medicine (DICOM) protocol, respectively. Based on digital imaging techniques, the entire spectrum of digital image processing is now applicable to the study of medicine.
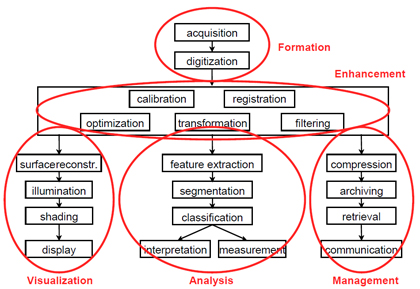
The commonly used term "medical image processing" means the provision of digital image processing for medicine. Medical image processing covers five major areas (see Figure 1):
- Image formation includes all the steps from capturing the image to forming a digital image matrix.
- Image visualization refers to all types of manipulation of this matrix, resulting in an optimized output of the image.
- Image analysis includes all the steps of processing, which are used for quantitative measurements as well as abstract interpretations of medical images. These steps require a-priori knowledge on the nature and content of the images, which must be integrated into the algorithms on a high level of abstraction. Thus, the process of image analysis is very specific, and developed algorithms can rarely be transferred directly into other domains of applications.
- Image management encompasses all the techniques that provide the efficient storage, communication, transmission, archiving and access (retrieval) of image data. A simple grayscale radiograph in its original condition may require several megabytes of storage capacity, and compression techniques are applied. The methods of telemedicine are also a part of image management.
- Image enhancement: in contrast to image analysis, which is also referred to as high-level image processing, low-level processing or image enhancement denotes manual or automatic techniques, which can be realized without a-priori knowledge on the specific content of images. This type of algorithm has similar effects regardless of what is shown in an image.
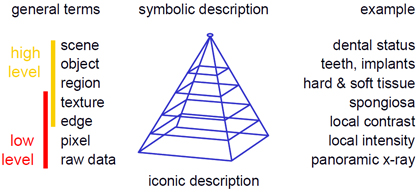
The complexity of an algorithm, the difficulty of its implementation, or the computing time required for image processing plays a secondary role in the distinction between low-level and high-level processing methods. Rather, the degree of abstraction of the a-priori knowledge is important for determining the distinction (Figure 2):
- The raw data level records an image as a whole. Therefore, the totality of all data pixels is regarded on this level.
- The pixel level refers to discrete individual pixels or points.
- The edge level represents the one-dimensional (1D) structures, which are composed of at least two neighboring pixels.
- The texture level refers to two-dimensional (2D) structures. On this level however, the delineation of the area's contour may be unknown.
- The region level describes 2D structures with a well-defined boundary.
- The object level associates textures or regions with a certain meaning or name, i.e., semantics is given on this level.
- The scene level considers the ensemble of image objects in spatial and/or temporal terms.
From an iconic (concrete) to a symbolic (abstract) description of images, information is gradually reduced. Methods of low-level image processing operate on the raw data as well as a pixel, edge, or texture levels, and thus at a minimal level of abstraction. Methods of high-level image processing include the texture, region, object, and scene levels. The required abstraction can be achieved by an increased modeling of a-priori knowledge.
With these definitions, a particular problem in high-level processing of medical images is immediately apparent: resulting from its complex nature, it is difficult to formulate medical a-priori knowledge such that it can be integrated directly and easily into automatic algorithms of image processing. This is referred to as the semantic gap, which means the discrepancy between the cognitive interpretation of a diagnostic image by the physician (high level) and the simple structure of discrete pixels, which is used in computer programs to represent an image (low level). In the medical domain, there are four main factors that make bridging the semantic gap difficult:
Low image quality: Most imaging modalities that are used in diagnostics or therapy are harmful to the human body. Therefore, images are taken with low energy or dose, and the signal to noise ratio is rather bad.
Heterogeneity of images: Medical images display organs or body parts. Even if captured with the same modality and following a standardized acquisition protocol, the shape, size, and internal structures of these objects may vary remarkably not only from patient to patient (inter-subject variation) but also among different views of a certain patient and similar views of the same patients at different times (intra-subject variation). In other words, biological structures are subject to both, inter- and intra-individual alterability. Thus, universal formulation of a-priori knowledge is impossible.
Unknown delineation of objects: Frequently, biological structures cannot be separated from the background because the diagnostically or therapeutically relevant object is represented by the entire image. Even if definable objects are observed in medical images, their segmentation is problematic because the shape or borderline itself is fuzzy or only partly represented. Hence, medically related items often can be abstracted at most on the texture level.
Robustness of algorithms: In addition to these inherent properties of medical images, which complicate their high-level processing, special requirements for reliability and robustness of medical procedures and, when applied in routine, image processing algorithms are also demanded in the medical area. As a rule, automatic analysis of images in medicine shouldn't provide wrong measurements. This means that images which cannot be processed correctly must automatically be rejected. Consequently, all images that are not rejected must be evaluated correctly. Furthermore, the number of rejected images must be quite small, since most medical imaging procedures are harmful and cannot be repeated just because of image processing errors.
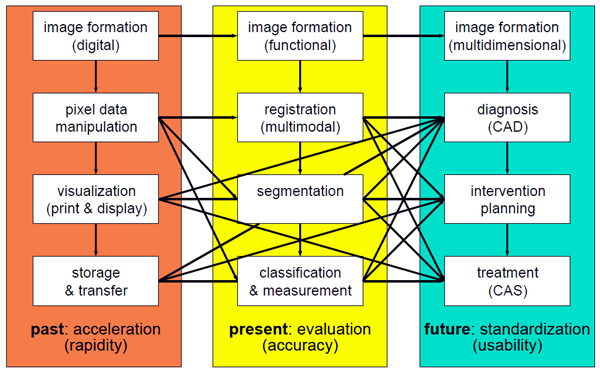
During the last decades, the paradigms of medical image processing have changed (Figure 3). In the 1980s, generation of digital images, appropriate management of image data as well as image enhancement and visualization were in the focus of research, and acceleration of the processing speed was needed. Since the 1990s, interpretation of medical images regarding registration, segmentation, classification, and reliable measurements has been developed, and evaluation of the algorithms has been focused on more. In the future, integration of medical image processing into the physicians' workflow will be fostered. High-level image processing will become part of diagnosis, intervention planning, and treatment; and further standardization will be required.
Because of this, medical image processing remains an exciting field of research and applications for health care, medical education, and biomedical research.
T. Deserno, "Medical Image Processing" in Optipedia, SPIE Press, Bellingham, WA (2009).