Designing high-performance micro-enzymatic biofuel cells
The US implantable medical devices market increases at an average rate of 8% every year and is expected to reach $73.9 billion by 2018.1 However, the size of conventional lithium-ion batteries is one of the limiting factors in the development of implantable medical microdevices. Miniaturized, self-sufficient bioelectronics powered by unconventional micropower may lead to a new generation of implantable, wireless, minimally invasive medical devices, such as pacemakers, defibrillators, drug-delivering pumps, sensor transmitters, and neurostimulators.2 Studies have shown that micro-enzymatic biofuel cells (EBFCs) are among the most intuitive candidates for in vivo micropower. As a ‘green’ power source, EBFCs can generate electricity via biocatalysis of glucose/oxygen, which are abundant in the human body.
 Recent advances in nanotechnology have opened up new opportunities for developing high-performance EBFCs. Graphene, a 2D sheet of carbon atoms with excellent conductivity, high surface area, and good mechanical stability, has shown promise for solar cells, supercapacitors, lithium-ion batteries, and fuel cells. State-of-the-art graphene-based EBFCs have also indicated that graphene can establish excellent electronic biocatalyst-electrode interaction.3, 4 In the past decade, our group has been developing 3D micro/nanostructures based on carbon microelectromechanical system platforms (C-MEMS) for different electrochemical and biological applications.5–18 In particular, we have integrated 2D graphene and graphene oxide with 3D carbon microstructures offering enlarged surface areas, superior physiochemical properties, enhanced redox reactions, and improved mass transport.
Recent advances in nanotechnology have opened up new opportunities for developing high-performance EBFCs. Graphene, a 2D sheet of carbon atoms with excellent conductivity, high surface area, and good mechanical stability, has shown promise for solar cells, supercapacitors, lithium-ion batteries, and fuel cells. State-of-the-art graphene-based EBFCs have also indicated that graphene can establish excellent electronic biocatalyst-electrode interaction.3, 4 In the past decade, our group has been developing 3D micro/nanostructures based on carbon microelectromechanical system platforms (C-MEMS) for different electrochemical and biological applications.5–18 In particular, we have integrated 2D graphene and graphene oxide with 3D carbon microstructures offering enlarged surface areas, superior physiochemical properties, enhanced redox reactions, and improved mass transport.
Modeling could play a vital role in optimizing the design of increasingly sophisticated devices, taking into account various factors regarding mass transport, electron transfer, and reaction kinetics. Recently, we have focused on establishing rigorously validated models in conjunction with experimental studies to develop advanced microbiofuel cells with optimized 3D C-MEMS-based EBFCs. We conducted a modeling study based on coupling mass transport, enzyme kinetics, and electron transfer with a COMSOL multiphysics module, which is a finite element analysis, solver, and simulation software package for various physics and engineering applications, especially coupled phenomena, or multiphysics.19
We considered the basic prototype design of an EBFC chip with 3D micropillars sitting on two sets of interdigitated fingers as the current collector. At steady state, competition between a higher enzyme reaction rate and a lower diffusion rate depleted glucose throughout the electrode surface, and accordingly the glucose concentration varied from the top to the bottom of the electrodes, inducing a nonuniform enzyme reaction rate. The chemical reactions are catalyzed by enzymes along the 3D electrodes.
Figure 1 shows the EBFCs' performance. For conventional cylindrical electrodes, we observed the lowest current density at the bottom of the electrode and the highest near the top corner of the electrode. For each set of fixed height electrodes, the maximum value for the generated current densities varied with the inter-electrode distance. A general design rule for the 3D microbiofuel cells can be inferred, that the current density is maximized when the electrode height is roughly double the distance between them (the so-called well width). Within the typical dimensions we could achieve by C-MEMS, the electrode arrays with height 200μm had the greatest power density at around 110μW/cm2, when the voltage was approximately 0.44V, which is sufficient to operate low-voltage CMOS integrated circuits.

In addition, we tested 2D graphene-encrusted 3D carbon microstructures as electrodes for micro-EBFCs (see Figure 2).20 To fabricate them, we combined top-down C-MEMS technology for the 3D micropillar arrays and bottom-up electrophoretic deposition (EPD) to co-deposit graphene/enzyme on the micropillar arrays for networked electrode interfaces. In brief, the C-MEMS-based 3D micropillar arrays were prepared by a two-step photolithography process using SU8-25 and SU8-100 (commonly used epoxy-based negative photoresists) followed by a pyrolysis step. EPD integrates graphene and enzyme composites onto the 3D micropillar array surface: see Figure 3(a). To evaluate the performance of the EBFC, we constructed a full cell by connecting bioanodes and biocathodes through an external variable resistor as shown in Figure 2 (right). In principle, glucose is catalyzed by glucose oxidase (GOx), produces gluconolactone and hydrogen ions, and generates electrons on the anode. On the cathode, the laccase catalyst reduces oxygen, and water is generated by the combination of electrons and hydrogen ions.

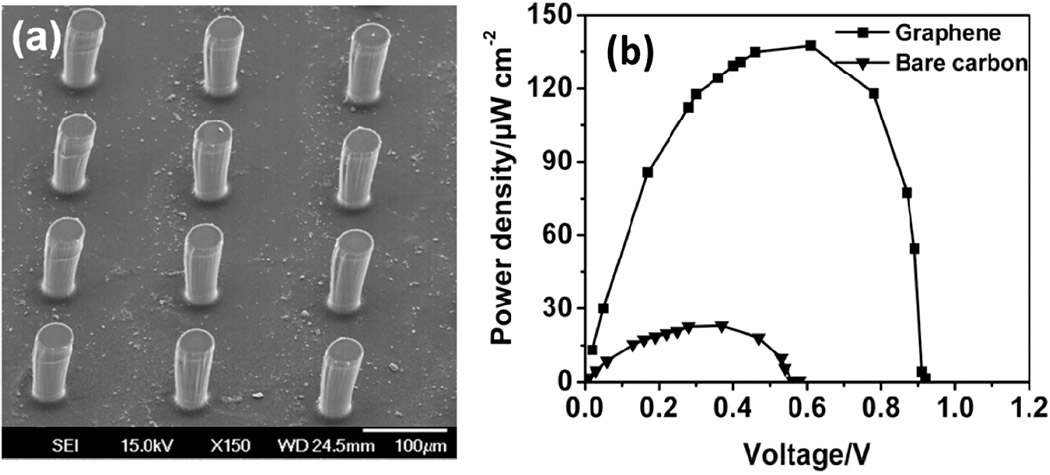
The graphene-based bioelectrode exhibited excellent electrochemical activity. Experimental results showed that the 3D graphene/enzyme-based EBFC generated a maximum power density of 136.3μWcm−2 at 0.59V, which is almost seven times that of a bare 3D-carbon-based EBFC: see Figure 3(b). The excellent performance was attributed to increased enzyme loading and more efficient electron transfer of the graphene-encrusted 3D micro-EBFC.
In summary, we have shown that 3D microstructures encrusted with 2D nanomaterials represent a promising platform for high-performance, micro-EBFCs for powering a new generation of implantable devices such as pacemakers or defibrillators. Our theoretical and experimental study of EBFCs has helped us to devise better ways of designing and using nanomaterials in practical micro-EBFC construction. In future work, we will continue to focus on modeling EBFC systems in a transient state within blood arteries and developing nano-enabled next-generation high-performance EBFCs. The ultimate goal for our research is to validate the theoretical findings through experimental performance of EBFCs and evaluate the efficiency of the actual system.
