Optipedia • SPIE Press books opened for your reference.
Fluorescence
Excerpt from Field Guide to Spectroscopy
In some atomic or molecular systems, light is absorbed by the system, undergoes some radiationless internal relaxation—either through vibrational states (vibrational relaxation), electronic states (internal conversion), or both—and then de-excites to the ground state by emitting a photon having a lower energy than the exciting photon. This process is called fluorescence.
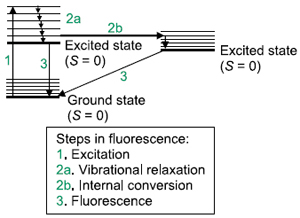
Fluorescence is a relatively fast process: 10-5-10-8 s. Fluorescence spectroscopy has three aspects: a plot of fluorescence versus wavelength of incident light; a plot of fluorescence versus the wavelength of emitted light; 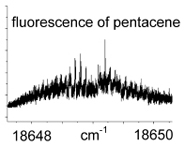 and the time frame in which the fluorescence is emitted. A diagram of the energy levels involved in a fluorescence process is called a Jablonski diagram. Because of the relaxation processes, fluorescence will usually be of a lower energy (i.e., lower frequency, higher wavelength) than the excitation light. High-resolution fluorescence spectra can show vibrational and, in the gas phase, rotational structure.
and the time frame in which the fluorescence is emitted. A diagram of the energy levels involved in a fluorescence process is called a Jablonski diagram. Because of the relaxation processes, fluorescence will usually be of a lower energy (i.e., lower frequency, higher wavelength) than the excitation light. High-resolution fluorescence spectra can show vibrational and, in the gas phase, rotational structure.
D. W. Ball, Field Guide to Spectroscopy, SPIE Press, Bellingham, WA (2006).
View SPIE terms of use.

